Beer's Lambert Law Equation
Beers Law and Lamberts law. Beers law states that the intensity of light decreases concerning _____ a Concentration b Distance c Composition d Volume.
B 7 The Beer Lambert Law Hl Youtube
Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a substance to be analyzed.
. It is used in analytical chemistry to measure the absorbance of several samples. Beer-Lambert Law demonstrates the linear relationship between the absorbance of light and the concentration of a substance. Check out the derivation of Beer-Lambert law.
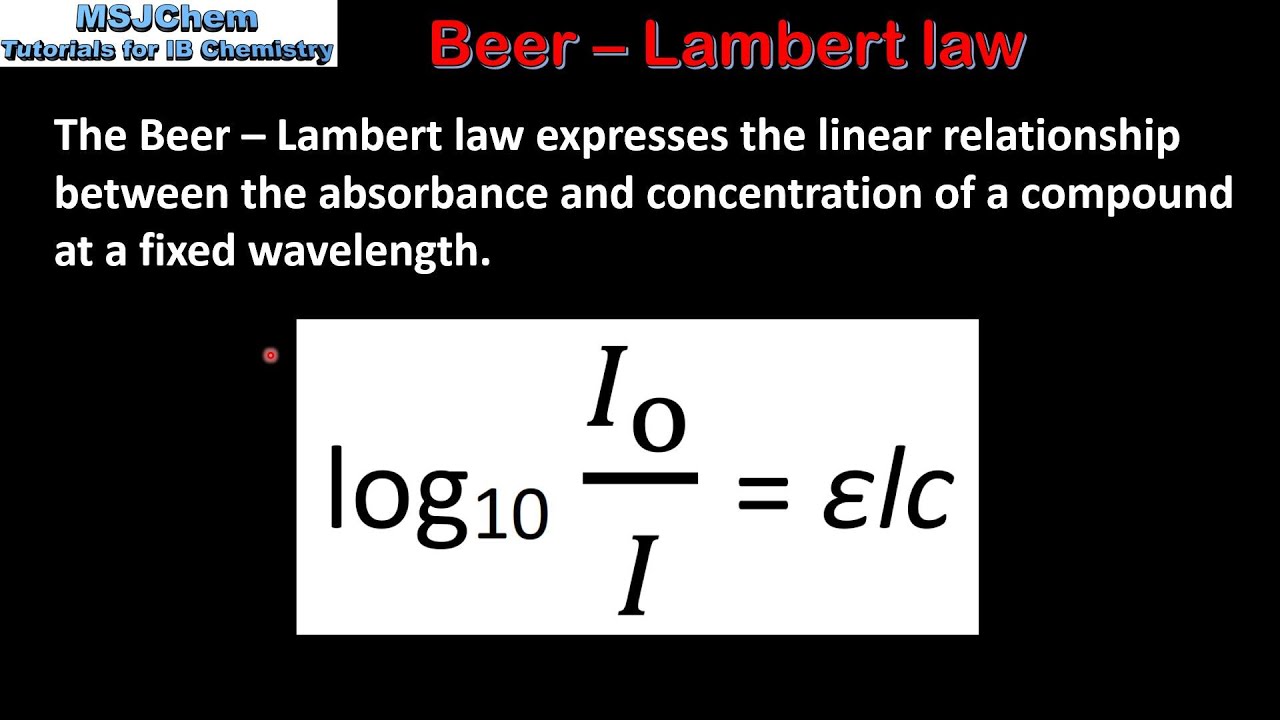
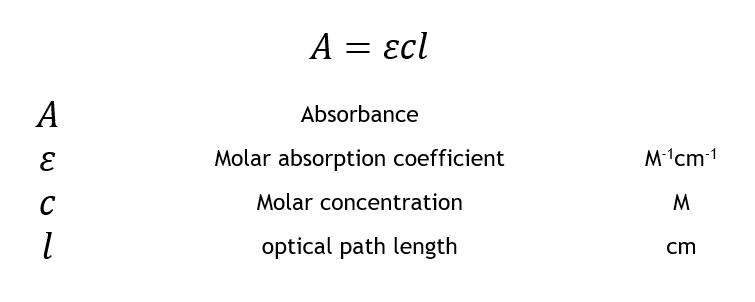
This expression relates the absorbance to the solute concentration in a given solution A eLc 1 where A is the compounds absorbance unitless e is the molar absortivity Lmolcm L is the path length cm and c is the compound concentration molL. MathrmA varepsilon bc nonumber. Absorbed as described by the Beer-Lambert equation also known as Beers Law.
Beers Law is also known as the Beer-Lambert Law the Lambert-Beer Law and the BeerLambertBouguer LawThe reason there are so many names is because more than one law is involved. As in the case of Lamberts law equation 9 may be transformed into log I Io 𝑐 𝑥 11. Newtons Law of Cooling.
Causes for deviation from Lambert-Beers law may be of chemical as well as instrumental origin At high drug or analyte concentrations typically 001 M causes deviations from linearity due to refractive index changes and because the close proximity of the absorbing molecules will affect their charge distribution and lead to alterations in their absorptivity. When a beam of monochromatic light is passed through a homogenous absorbing medium the rate of decrease of intensity of radiation with increase in the concentration c of absorbing species is directly proportional to the intensity I of the incident light radiation. -dIdc k I -dII k d c On integration of above equation -ln I k c.
The Beer-Lambert law known by various names such as the Lambert-Beer law Beer-LambertBouguer law or the Beers law. The colorimeter is usually used to measure the concentration of a known solute in a given solution with the help of the Beer-Lambert law. Normality N - grams active soluteliters of solution Molality m - moles of solutemass of solvent not mass of solution.
This page takes a brief look at the Beer-Lambert Law and explains the use of the terms absorbance and molar absorptivity relating to. Since the concentration path length and molar absorptivity are all directly proportional to the absorbance we can write the following equation which is known as the Beer-Lambert law often referred to as Beers Law to show this relationship. Mass Concentration kgm 3 or gL - mass of solutevolume of solution.
A reagent termed the titrant or titrator is prepared as a standard solution of known concentration and volume. Newtons law of cooling defines the rate at which an exposed body changes temperature by radiation which is roughly equal to the difference in temperature between the item and its surroundings provided. Newton was the first to analyze the relationship between the heat lost by a body in a certain enclosure and its temperature systematically.
The titrant reacts with a solution of analyte. Beer-Lambert law explains the relationship between absorbance path length and concentration of a given material. Beer-lamberts law Beers Law.
Beer-lambert law describes the link between the attenuation of light through. There is a fee for seeing pages and other features. Das Gesetz bildet die.
The term is used in many technical areas to quantify the. Beer-Lambert Law is a combination of two laws. The colorimeter was invented in the year 1870 by Louis J Duboscq.
The two laws may be combined to write. Other Names for Beers Law. When the concentration c is expressed in mol L b is called the molar absorption co-efficient.
This is known as the Beers law. Molarity M - moles of soluteliters of solution not solvent. According to this law theoretically a calibration curve generated by observing the response of the instrument in terms of the liquids absorbance for its different concentrations looks like a straight line.
Absorbance is defined as the logarithm of the ratio of incident to transmitted radiant power through a sample excluding the effects on cell walls. Lamberts law states that the intensity of light decreases concerning _____ a Concentration b Distance c Composition d Volume. It refers to a device which helps specific solutions to absorb a particular wavelength of light.
The Beer-Lambert law relates the attenuation of light to the properties of the material through which the light is traveling. Mass Percent - mass solutemass solution x 100 mass units are the same unit for both solute and solution. Basically Pierre Bouger discovered the law in 1729 and published it in Essai DOptique Sur La Gradation De La Lumière.
Alternatively for samples which scatter light absorbance may be defined as the negative logarithm of one minus absorptance as measured on a uniform sample. Das lambert-beersche Gesetz oder bouguer-lambert-beersche Gesetz beschreibt die Abschwächung der Intensität einer Strahlung in Bezug zu deren Anfangsintensität bei dem Durchgang durch ein Medium mit einer absorbierenden Substanz in Abhängigkeit von der Konzentration der absorbierenden Substanz und der Schichtdicke. Papers from more than 30 days ago are available all the way back to 1881.
This law of analytical chemistry states that the relationship between the concentration of any solution and its absorbance is linear. The representation of Beer Lamberts law is given as A ABC.

Beer Lambert Law Labster Theory

Beer S Law Equation And Example

How To Find Molar Absorptivity Using The Beer Lambert Law Chemistry Study Com

Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
No comments for "Beer's Lambert Law Equation"
Post a Comment